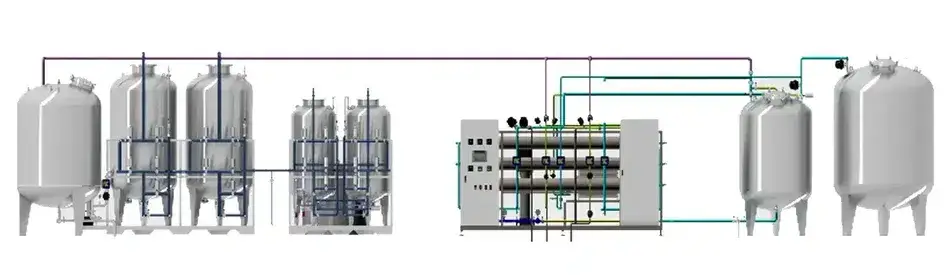
We specialize in the design and implementation of purified water systems primarily developed for pharmaceutical manufacturing, while also adaptable to related industries such as biotechnology, food processing, and cosmetics. Our water treatment solutions are engineered to meet GMP standards for clean utility applications and are designed with flexibility to accommodate diverse production environments. Our main pharmaceutical water systems include Purified Water (PW), Water for Injection (WFI), and Pure Steam (PS) generation, each developed to ensure quality, safety, and process reliability. Besides the purified water systems we offer, we also provide supporting systems such as pretreatment units, storage and distribution solutions, formulation systems, and CIP (Cleaning in Place) systems. Each water treatment system is engineered with precision, supported by automation control and validation documentation, to meet the strict standards of pharmaceutical production environments.

We provide full lifecycle support for pharmaceutical purified water systems, from project planning to long-term service. Our approach is built around clear communication, personalized solutions, and close follow-up after installation.
We help analyze user requirements (URS), assess technical feasibility, and plan the project with a focus on practicality and compliance.
Our team ensures proper design confirmation, smooth installation and commissioning, validation support, and on-site staff training.
We offer a quick response service, long-term technical assistance, and a one-year warranty for key components.
In addition to regular maintenance, we also provide system upgrades and personalized validation support. We stay in touch through follow-up visits and inspections to help reduce potential failures caused by poor maintenance and to support clients in building more efficient management systems. This hands-on service model has helped us set a higher standard in the industry.


PW/WFI pre-treatment system for eliminating contaminants, particles, turbidity and initial hardness from raw water prior to the water purification process

Producing pharmaceutical grade purified water in full compliance with CP, USP and EP guidelines

Purified water and WFI must be stored and distributed under strict hygienic conditions to maintain their quality until the point of use.

Multiple effect stills for producing Water for Injection (WFI) in accordance with CP, USP and EP pharmacopoeias

Multiple effect stills for producing Water for Injection (WFI) in accordance with CP, USP and EP pharmacopoeias

Stainless steel pharmaceutical storage tanks for storing sterile water for injection

Storage and distribution of pharmaceutical grade purified water
WEMAC provides a range of water solutions for pharmaceutical use, including multi-effect water distillers, pure water generation and storage systems, pure steam generators, and seawater desalination systems.
WEMAC products are designed and manufactured in accordance with GMP requirements for the pharmaceutical industry, ensuring that the water produced meets the highest standards of purity and quality.
Yes, WEMAC offers customizable options such as the customizable CIP (Cleaning In Place) module and the Integrated Purified Water & WFI Storage System, which can be tailored to meet the unique requirements of different pharmaceutical processes.
WEMAC offers multi-language capabilities and ODM services, allowing them to cater to the diverse needs of their global customer base by providing support in various languages and offering design-based customization insights.
Please take a minute to confirm your process requirements by completing the MSR™ Microsphere Refiner online questionnaire. Our team will help you select the most suitable solution for purified water, WFI, and pure steam generation.